Atom
History of atomic theory
In philosophy
Dalton's law of multiple proportions
Kinetic theory of gases
Brownian motion
Discovery of the electron
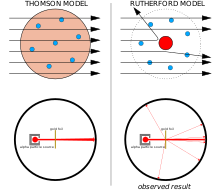
Discovery of the nucleus
Discovery of isotopes
Bohr model
The Schrödinger model
Discovery of the neutron
Fission, high-energy physics and condensed matter
Structure
Subatomic particles
Nucleus
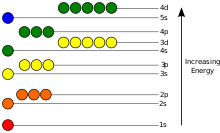
Electron cloud
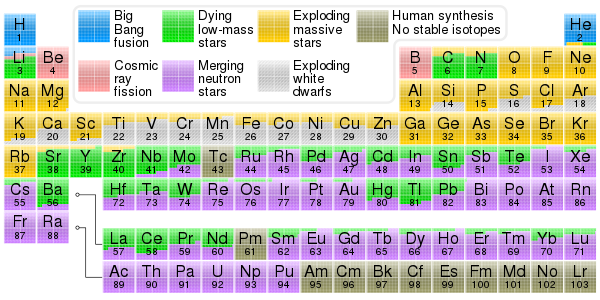
Properties
Nuclear properties
Mass
Shape and size
Radioactive decay
The most common forms of radioactive decay are:[82][83]
- Alpha decay: this process is caused when the nucleus emits an alpha particle, which is a helium nucleus consisting of two protons and two neutrons. The result of the emission is a new element with a lower atomic number.
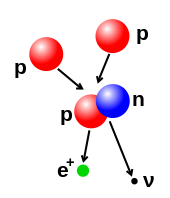
- Beta decay (and electron capture): these processes are regulated by the weak force, and result from a transformation of a neutron into a proton, or a proton into a neutron. The neutron to proton transition is accompanied by the emission of an electron and an antineutrino, while proton to neutron transition (except in electron capture) causes the emission of a positron and a neutrino. The electron or positron emissions are called beta particles. Beta decay either increases or decreases the atomic number of the nucleus by one. Electron capture is more common than positron emission, because it requires less energy. In this type of decay, an electron is absorbed by the nucleus, rather than a positron emitted from the nucleus. A neutrino is still emitted in this process, and a proton changes to a neutron.
- Gamma decay: this process results from a change in the energy level of the nucleus to a lower state, resulting in the emission of electromagnetic radiation. The excited state of a nucleus which results in gamma emission usually occurs following the emission of an alpha or a beta particle. Thus, gamma decay usually follows alpha or beta decay.













No comments:
Post a Comment